What is Graphene?
Graphene is a “super-material,” with the potential to change history like the bronze, iron, steel or silicon of past eras.
It is a two-dimensional material, only one atom thick, consisting of sheets of carbon atoms bonded in a hexagonal pattern.
Scientists first hypothesised its existence in the 1940s, but it was not until 2004 that Konstantin Novoselov and Andre Geim were able to create it from graphite and study its properties. They won a Nobel Prize in 2011 for their work.
Since then, graphene research has exploded with a vast number of patents taken out as markets and industries prepare for a disruptive Age of Graphene.
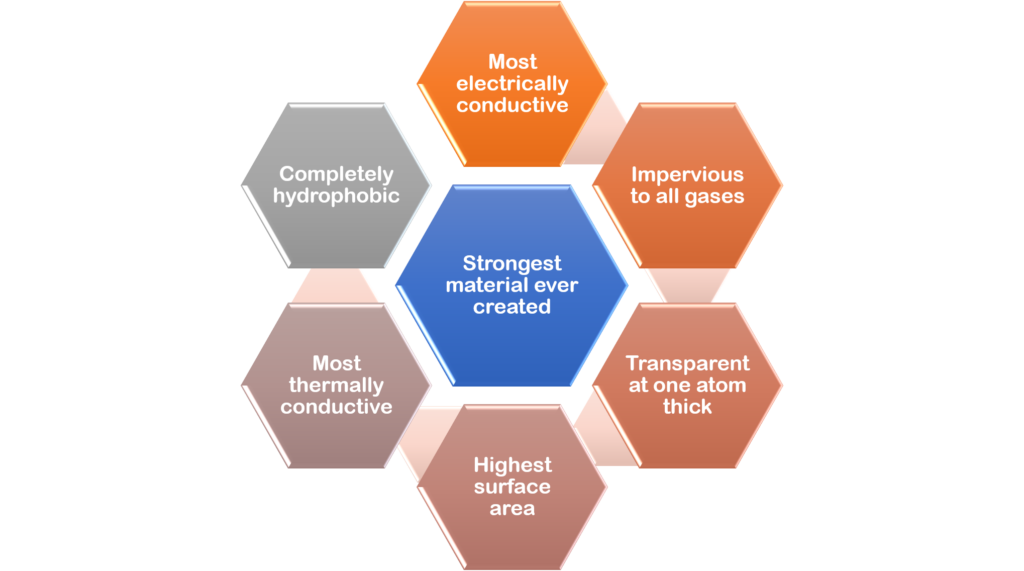
What are graphene’s extraordinary properties?
- Graphene is the thinnest material available for use in commercial applications. It is only one carbon atom thick.
- Graphene is the strongest and hardest materials in the world. It is harder than diamonds and 200 times stronger than steel of the same thickness but, notwithstanding this, graphene is very flexible and will not break.
- Graphene has amazing optical properties. A one atom thick sheet will absorb only 2.3% of visible light, making it transparent. Graphene can also absorb up to 40% of light in the far infrared and microwave frequency ranges, confirming that it could be ideal for terahertz and photonics applications.
- Graphene is exceptionally light and stretchable. Graphene weighs only 0.77 milligrams per square metre and is stretchable up to 20% of its initial length. It has the largest volume-to-surface area ratio of any material.
- Graphene is completely impermeable. Even helium atoms cannot pass through it.
- Graphene has high thermal conductivity. It is a perfect thermal conductor (over 5,000 W/mK), being five times the conductivity of graphite. Graphene conducts heat in all directions, that is, it is an isotropic conductor.
- Graphene has amazing electronic properties. It has the highest electrical density of all materials (1,000,000 times that of copper) and conducts electricity close to the speed of light with virtually no resistance and also has the highest intrinsic mobility (100 times that of silicon). It has a lower resistivity than any other know material at room temperature.
- Graphene is chemically inert and does not readily react with other atoms. However, it can “absorb” different atoms and molecules, leading to changes in its properties. It can be functionalised by several different chemical groups, resulting in different materials such as GO and fluorinated graphene.
- Graphene is also capable of self-healing and repairing holes in its sheets when exposed to free molecules containing carbon.
What is graphene used for?
Actual and potential applications of graphene continue to grow, limited only by current research, development and testing.
Current applications for graphene already in play include the infusion of electrical conductivity in plastics and glass and the production of composite materials such as carbon fibres, plastics, geo-fabrics, coatings and paints with impermeable and anti-corrosion qualities.
Graphene has the potential to create the next generation of electronics currently limited to science fiction: faster transistors, semiconductors, bendable phones and flexible wearable electronics. It could be a part of your wall paint – simply flick a button and a section becomes a screen. Even
conductive inks, tattooed into your arm and powered by your own body’s heat and movement, will be possible. With new uses being dreamed up for graphene every day, from indestructible condoms to filters that
can remove pollution from the atmosphere, it’s good to know that the processes for creating graphene have a low environmental impact.
There is a vast range of other potential applications including:
- Electronics
- Energy
- Water treatment
- Construction
- Aerospace engineering
- Biotech and Medtech
- Chemical processing
Two applications, addressing some of the world’s greatest challenges, with enormous market potential, and where Ionic is focused are water filtration and energy storage.